According to: http://en.wikipedia.org/wiki/Thermal_radiation
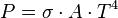
Where P is energy radiated and T is temperature in Kelvin.
300F = 422K
600F = 588K
588^4/422^4=3.77
If I have this right, a stove at 600F is radiating 3.77 times the heat of one at 300F.
I've noticed my stove doesn't consume that much more fuel at 600F than at 300F. Lately, instead of trying to maintain a long, low overnight burn, I run the stove even hotter (650F) at bedtime when I leave the main living space. While I'm sure the room gets uncomfortably warm as I sleep, it's around 68-70F when I wake up. The stove is under 200F and the glass is just slightly hazy. I find that dumping heat into the room quickly gives me the same result in the morning with less wood and a cleaner stove. Any thoughts?
Where P is energy radiated and T is temperature in Kelvin.
300F = 422K
600F = 588K
588^4/422^4=3.77
If I have this right, a stove at 600F is radiating 3.77 times the heat of one at 300F.
I've noticed my stove doesn't consume that much more fuel at 600F than at 300F. Lately, instead of trying to maintain a long, low overnight burn, I run the stove even hotter (650F) at bedtime when I leave the main living space. While I'm sure the room gets uncomfortably warm as I sleep, it's around 68-70F when I wake up. The stove is under 200F and the glass is just slightly hazy. I find that dumping heat into the room quickly gives me the same result in the morning with less wood and a cleaner stove. Any thoughts?



