I'm lifting this from another thread. There's been some great discussion about this topic, and I think it deserves a thread of its own.
I've been guilty of promoting the value of really dry wood for gasifiers. While it's necessary to get them started, it may be overrated. I did some detailed system efficiency calculations and found virtually no difference between wood at 30% and wood at 20%. Based on that and many related posts, I decided to do a real (gasp) analysis of the energy loss from moisture in wood. Here it is - let the fur begin flying.
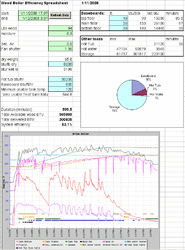
From the initial post, we have 94 lbs wood at average 30% moisture = 65.8 pounds ‘bone’ dry at 8600 BTU/lb = 565880 potential BTU
Calculating the energy lost to the water in the wood:
It takes 1 btu per pound to raise water 1 degree. It takes 970 BTU to boil one pound of water that’s at 212 degrees. It takes .5 BTU per pound to raise steam 1 degree.
We have about 30 pounds of water in our wood. If the wood starts out at 70 degrees and the steam goes up the flue at 600 degrees, it will require the following energy which will be lost to the system:
30 lbs water, 60 to 212 degrees = 4560 BTU
30 lbs water to 30 lbs steam = 29,100 BTU
30 lbs steam 212 to 600 degrees = 5820 BTU
Total loss = 39,380 BTU at 30% moisture. This represents 7% of the energy available in the wood itself. Thus, I’d expect a 7% loss compared to bone dry wood. Wood at 20% should represent a 4.6% loss.
The theoretical difference between the energy loss due to water at 30% vs. water at 20% is less than 2.5% of the available energy, assuming the same piece of wood in both cases.
Not a big number. I should have done the math earlier.
Of course, if you flip that around, this 7% number could account for a sizable majority of your combustion efficiency losses if you’re somewhere near 90%. From a system level, it’s not such a big deal. According to these numbers, I need to burn another tenth of a cord if it’s 30% instead of 20%.
Based on a post by slowzuki in another thread, I'm also calculating non-water flue gas losses.
Air has a specific heat of .24BTU/lb. It takes about six pounds of air to burn one pound of wood, although the boiler fan may drive much more than that. Making a wild assumption that the specific heat of all the non-water flue gas is about that value, we can calculate heat loss up the flue.
For the original example, 6 pounds of air per pound of wood = 455 pounds of flue gas at 600 degrees = 59,000 BTU of non-water flue gas loss for another 10%. At this point, we're down to around 80% depending on the unburned fuel losses. I actually got 56% measured, so I'm losing energy elsewhere.
Here's the beginning of a breakdown of losses:
Starting BTU value of wood: 565,880
Water related losses at 30% moisture: 39,380
Non-water flue gas loss at 600 degrees: 59,000
At this point, we're down to 467,500 BTUs or 82% of the original potential.
I've been guilty of promoting the value of really dry wood for gasifiers. While it's necessary to get them started, it may be overrated. I did some detailed system efficiency calculations and found virtually no difference between wood at 30% and wood at 20%. Based on that and many related posts, I decided to do a real (gasp) analysis of the energy loss from moisture in wood. Here it is - let the fur begin flying.
From the initial post, we have 94 lbs wood at average 30% moisture = 65.8 pounds ‘bone’ dry at 8600 BTU/lb = 565880 potential BTU
Calculating the energy lost to the water in the wood:
It takes 1 btu per pound to raise water 1 degree. It takes 970 BTU to boil one pound of water that’s at 212 degrees. It takes .5 BTU per pound to raise steam 1 degree.
We have about 30 pounds of water in our wood. If the wood starts out at 70 degrees and the steam goes up the flue at 600 degrees, it will require the following energy which will be lost to the system:
30 lbs water, 60 to 212 degrees = 4560 BTU
30 lbs water to 30 lbs steam = 29,100 BTU
30 lbs steam 212 to 600 degrees = 5820 BTU
Total loss = 39,380 BTU at 30% moisture. This represents 7% of the energy available in the wood itself. Thus, I’d expect a 7% loss compared to bone dry wood. Wood at 20% should represent a 4.6% loss.
The theoretical difference between the energy loss due to water at 30% vs. water at 20% is less than 2.5% of the available energy, assuming the same piece of wood in both cases.
Not a big number. I should have done the math earlier.
Of course, if you flip that around, this 7% number could account for a sizable majority of your combustion efficiency losses if you’re somewhere near 90%. From a system level, it’s not such a big deal. According to these numbers, I need to burn another tenth of a cord if it’s 30% instead of 20%.
Based on a post by slowzuki in another thread, I'm also calculating non-water flue gas losses.
Air has a specific heat of .24BTU/lb. It takes about six pounds of air to burn one pound of wood, although the boiler fan may drive much more than that. Making a wild assumption that the specific heat of all the non-water flue gas is about that value, we can calculate heat loss up the flue.
For the original example, 6 pounds of air per pound of wood = 455 pounds of flue gas at 600 degrees = 59,000 BTU of non-water flue gas loss for another 10%. At this point, we're down to around 80% depending on the unburned fuel losses. I actually got 56% measured, so I'm losing energy elsewhere.
Here's the beginning of a breakdown of losses:
Starting BTU value of wood: 565,880
Water related losses at 30% moisture: 39,380
Non-water flue gas loss at 600 degrees: 59,000
At this point, we're down to 467,500 BTUs or 82% of the original potential.


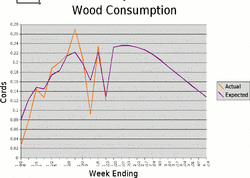
 Certainly using a volumetric measurement (cord) for a wildly variable density solid like wood is enough as it is to make even the most jaded engineer keel over. But I would never weigh all the wood burned in a season either. It requires enough effort just to do everything else.
Certainly using a volumetric measurement (cord) for a wildly variable density solid like wood is enough as it is to make even the most jaded engineer keel over. But I would never weigh all the wood burned in a season either. It requires enough effort just to do everything else.