RenovationGeorge said:
Could you please help me understand how you calculated overall efficiency? Is that the percent of the theoretical energy content in the wood, based on the amount of wood fiber you estimate to be in the wood, without heat of vaporization subtracted out?
George, you do realize that I didn't do this study, don't you? I didn't decide anything, Dr. Jay Shelton of Shelton Research, Inc. did all the deciding back in 1986.
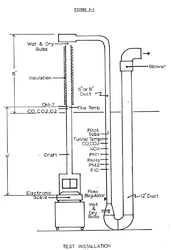
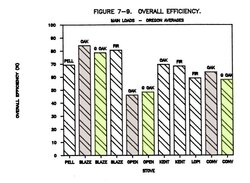
The overall efficiency was determined by using information gathered from numerous sensors strategically placed in the flue (see diagram below). This is called the the "stack loss method", and is used throughout the industry, both in the design phase and during testing. It is the custom in North America to use the higher heating value (probably to make the stoves appear more energy efficient). This leads to a lot of confusion as well as yielding a figure that no one is interested in knowing. We want to know how much heat is coming into the room for every ounce of wood we burn. The industry is usually not giving us the correct answer, and we have no sure way of knowing when they are.
Shelton Research used a much more realistic method to determine overall heating efficiency by subtracting
all energy loses (chemical energy, sensible heat lost up the flue, and latent heat losses through evaporation). This method correlates highly with heat output testing of wood stoves done in actual calorimeter rooms, so it is considered to be very accurate. The formulas Shelton used were:
Overall efficiency = (wood energy input minus sensible heat loss minus chemical energy loss minus latent heat lost) divided by wood energy input
Combustion efficiency = (wood energy input minus chemical energy loss) divided by wood energy input
Heat transfer efficiency = (wood energy input minus sensible heat loss minus chemical energy loss minus latent heat lost) divided by (wood energy input minus chemical energy loss)
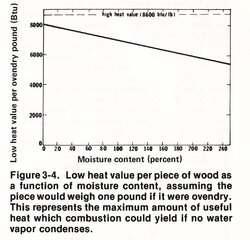
By using the lower heating value (values in the chart I posted above) instead of the higher heating value (usually used in North America) for expressing wood energy, he was able to compute the latent heat loss from unrecondensed water vapor leaving the flue based on the assumption of complete combustion without any effect on the accuracy of the data.
It is all here on page 27:
http://www.arb.ca.gov/research/apr/past/a3-122-32.pdf
Have fun with it... it's very long and detailed, but looking at your profile page, I'm sure you can handle it.
BTW thanks for sharing my curiosity about all this, and for expressing your interest in public here. May I call you Curious George?

Just remember that none of this will rock your world when it comes to actually using your stove when you finally decide which one you want. That's art, not science. Guys like Dennis ("Backwoods Savage") could give two shits about all this stuff, and they burn better and smarter than 95% of the young guys with all the "answers" (Hint: I ain't a young guy). This stuff
may help you make the best decision on what stove to buy, or how to install the best chimney (be nice to have Shelton's drafting system, eh?), etc. That's all I hope to do by discussing this all here.
And feel free to blast away at my statements. I'd hate to have erroneous statements or calculations I may make go unchallenged. In fact, I welcome as much fire as you can hit me with. Makes me think harder and staves off the Alzheimer's for a while longer.







 Just remember that none of this will rock your world when it comes to actually using your stove when you finally decide which one you want. That's art, not science. Guys like Dennis ("Backwoods Savage") could give two shits about all this stuff, and they burn better and smarter than 95% of the young guys with all the "answers" (Hint: I ain't a young guy). This stuff may help you make the best decision on what stove to buy, or how to install the best chimney (be nice to have Shelton's drafting system, eh?), etc. That's all I hope to do by discussing this all here.
Just remember that none of this will rock your world when it comes to actually using your stove when you finally decide which one you want. That's art, not science. Guys like Dennis ("Backwoods Savage") could give two shits about all this stuff, and they burn better and smarter than 95% of the young guys with all the "answers" (Hint: I ain't a young guy). This stuff may help you make the best decision on what stove to buy, or how to install the best chimney (be nice to have Shelton's drafting system, eh?), etc. That's all I hope to do by discussing this all here.