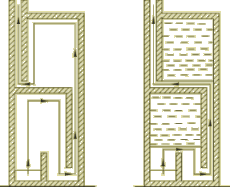
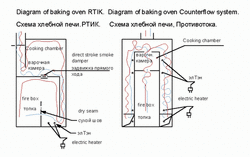
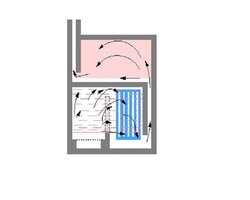
I
ISeeDeadBTUs
Guest
[quote author="slowzuki" date="1233785178"]Strange how a glowing mass that fills the whole stove doesn't throw much heat.[/quote]
A bed of coals the size we can end up with in a hydronic, if placed in a wood stove, would drive everyone out of the house or have us open the windows. But without FLAME in these 'Gassers' you just won't get the quality heat production.
What's the technical X-Plain-nation for dat??
A bed of coals the size we can end up with in a hydronic, if placed in a wood stove, would drive everyone out of the house or have us open the windows. But without FLAME in these 'Gassers' you just won't get the quality heat production.
What's the technical X-Plain-nation for dat??