zrtmatos
Feeling the Heat
I replaced a pellet stove with 34K output and no OAK with a stove that had slightly more output, 38K but installed an OAK with it. Big difference in cutting down air leaking in through an adjacent room to the stove. This room has newer windows and the leaks of air found their way in without the OAK. I can attest to it working. I probably could have not changed stoves and just installed an OAK. Just do the OAK install in the first place.





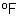 and vent gasses?
and vent gasses? ,
,  in with the smilies.
in with the smilies. 