Posting for the TEG crowd out there: https://www.sciencemag.org/news/2021/08/cheap-material-converts-heat-electricity
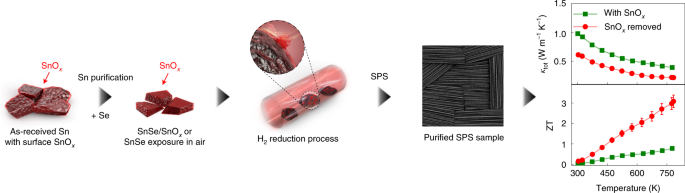
I believe the zT 'figure of merit' they quote as ~3.1 for this new polycrystaline tin selenide material means it would be roughly three times as efficient as the current standard bismuth telluride - which I typically see quoted around 1.0. So assuming they can keep other factors relatively in check, this might be an interesting material for near-future development.
Article gets a little out in the weeds by the end saying people might hook these up to water heaters to create electricity. Well, I guess if you have a water heater where you don't actually want hot water! Otherwise, you'd be much better off to insulate the water heater and put this somewhere you actually have waste heat!
I believe the zT 'figure of merit' they quote as ~3.1 for this new polycrystaline tin selenide material means it would be roughly three times as efficient as the current standard bismuth telluride - which I typically see quoted around 1.0. So assuming they can keep other factors relatively in check, this might be an interesting material for near-future development.
Article gets a little out in the weeds by the end saying people might hook these up to water heaters to create electricity. Well, I guess if you have a water heater where you don't actually want hot water! Otherwise, you'd be much better off to insulate the water heater and put this somewhere you actually have waste heat!